
Research Goal:
Whether from a viral infection or some sort of cellular dysfunction, cytosolic dsRNA is an indication that something is not right in the cell. Research in the Ramsey Lab centers on a family of innate immune receptors called the RIG-I-like receptors (RLRs) which are responsible for sensing the presence of cytosolic dsRNA and initiating an innate immune response. Our approach to studying the RLRs is inspired by comparative immunology. We believe that by biophysically characterizing RLRs from many different species we will discover fundamental aspects of their functions as well as identify plasticity in their mechanisms which have allowed them to evolve and occupy different ecological niches. By combining our molecular biophysical investigations with cell-based functional assays, our work will provide a holistic perspective on RLR function and inform on the design of therapeutics from novel antivirals to vaccine adjuvants and cancer immunotherapies.
Why should we study RLRs?
RNA viruses comprise some of the most significant health threats across the world for both humans and animals. The innate immune system constitutes the body’s first line of defense to invasions by these pathogens. The RIG-I-like receptors (RLRs) are a family of pattern recognition receptors which are responsible for sensing cytosolic double-stranded RNA (dsRNA), a highlight of viral infection, and initiating an innate immune response. Proper function of RLRs is required for a robust initial defense against viral infection. Furthermore, RLRs are a common target for vaccine adjuvant design and are even emerging as potential targets for therapeutics which sensitize certain tumors to novel immune checkpoint inhibitors. It is critical that we understand the precise biophysical mechanisms underlying RLR function in order to inform the design of therapeutics which target RLRs.
What do we know about how RLRs function?
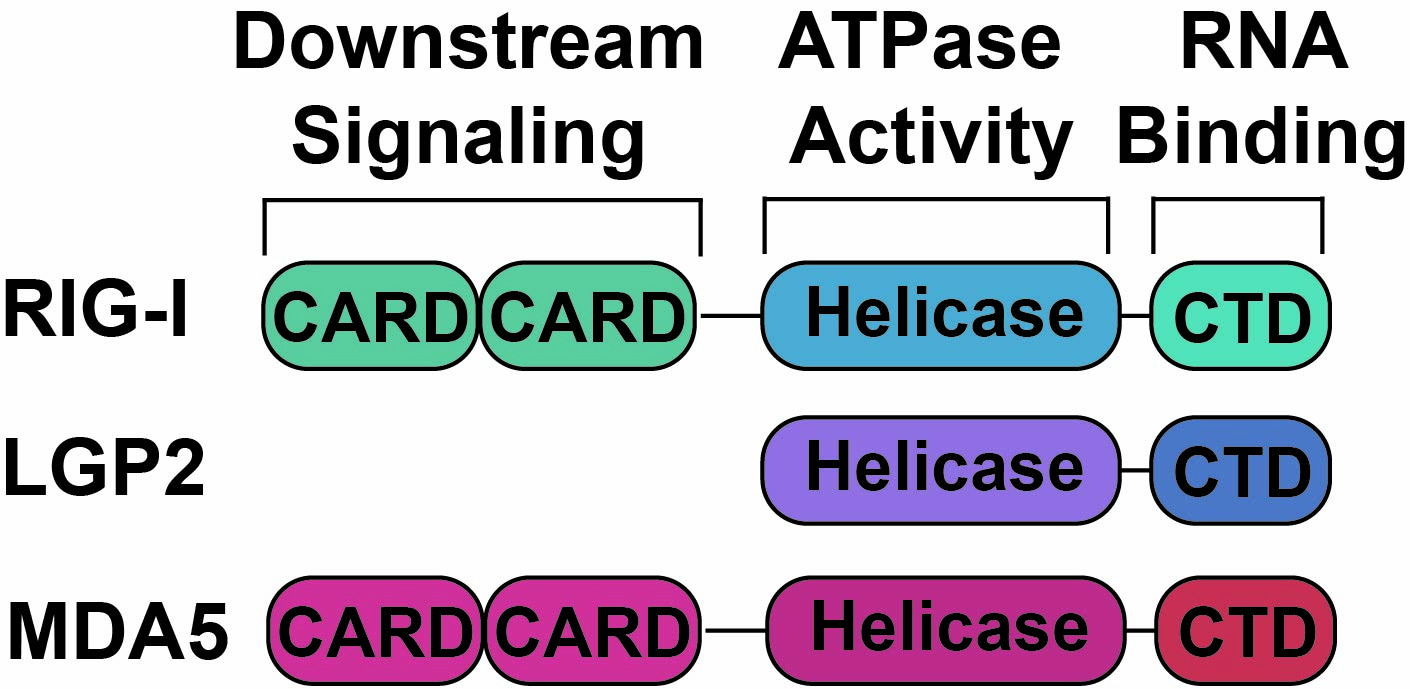
RLRs are a family of three ATP-dependent RNA helicases, RIG-I, MDA5, and LGP2, which share an overall conserved domain architecture but possess non-redundant functions. All RLRs have a central helicase domain (Hel) and C-terminal domain (CTD) which, together, are responsible for binding to pathogenic dsRNAs. RIG-I and MDA5 additionally have two N-terminal caspase activation and recruitment domains (CARDs) which are the mediators of downstream signaling. Interestingly, LGP2 lacks the CARDs and is therefor unable to signal on its own but is believed to be a regulator of RIG-I and MDA5.
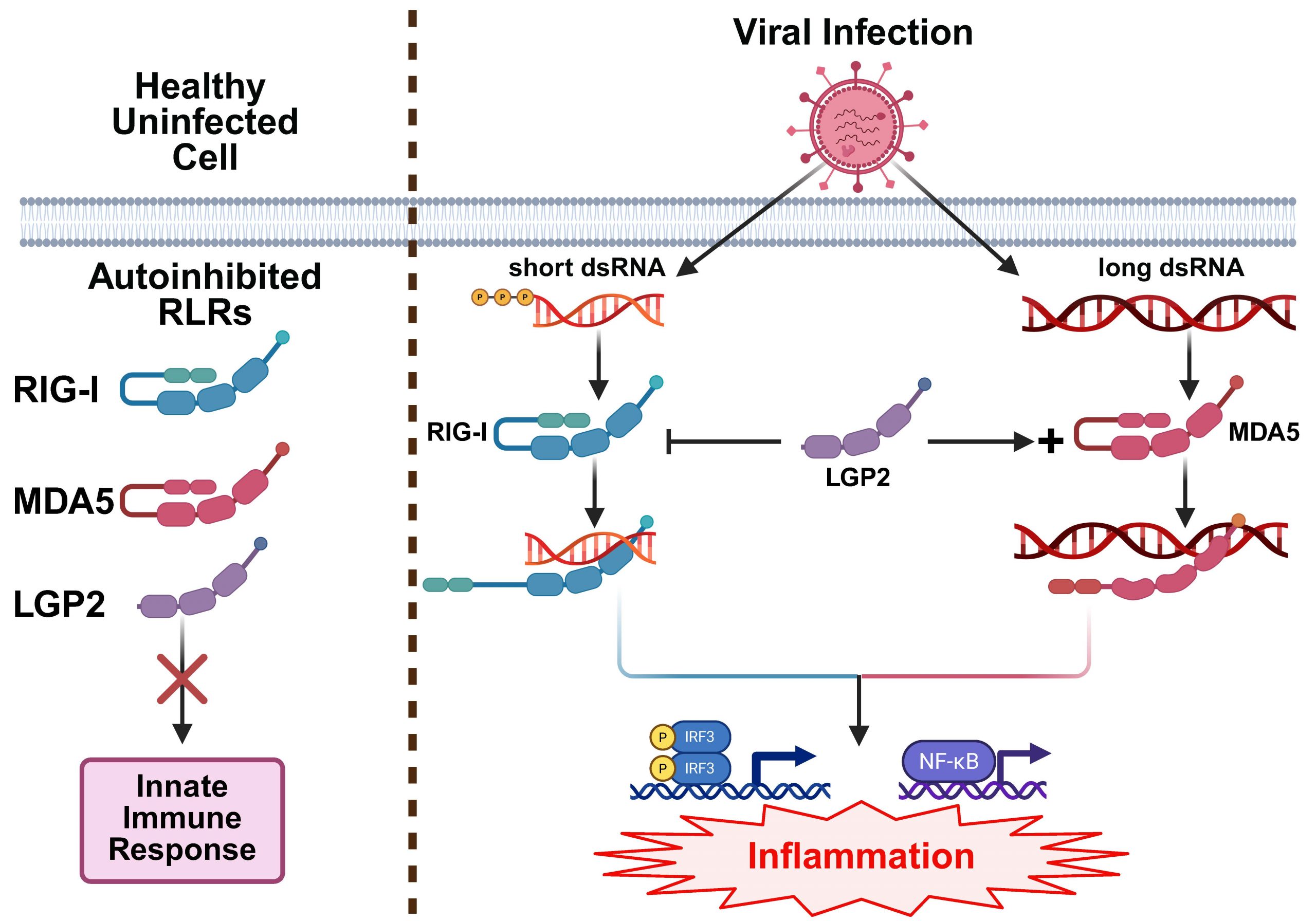
In uninfected cells, RIG-I and MDA5 exist in auto-inhibited conformations wherein their CARDs are inaccessible for binding to downstream partners. Upon encountering dsRNA, RIG-I and MDA5 undergo large-scale conformational changes which expose their CARDs, allowing them to bind to downstream effectors and initiate an innate immune response characterized by the production of pro-inflammatory cytokines.
RLR Signaling
How do we study RLRs in the Ramsey Lab?
We utilize a unique approach to understanding the fundamental biology of RLR function inspired by comparative immunology wherein we study RLRs not only from humans but also from avian and scavenger species. Furthermore, we combine rigorous biophysical characterization with in-cell functional assays to relate in vitro results to changes in RLR behavior in cells. Our comparative immunological approach along with our emphasis on connecting biophysical properties to cellular function will reveal universal mechanisms of signaling by these receptors and identify unique adaptations specific to a particular ecological niche. Research in the Ramsey Lab is concentrated in three main areas:
AREA 1: Human RLRs
Since it has been shown that RLRs are multi-domain proteins regulated by inter-domain interactions and large-scale conformational changes, we are interested in investigating the functional role of human RLR (hRLR) conformational changes during dsRNA sensing and subsequent signaling. By utilizing structural, kinetic, and thermodynamic approaches, we are attempting to answer critical questions regarding the molecular mechanisms underlying fundamental RLR biology in humans.
AREA 2: Avian RLRs
While humans possess three RLRs, there are certain clades of birds which have lost the gene for certain family members. The Galliform birds (which include chickens, turkeys and other land-fowl) have lost the RIG-I gene while the Ciconiiformes (storks) and Gruiformes (rallids) have lost a functional MDA5 gene. It is unclear how the remaining family members have evolved mechanistically to accommodate this smaller RLR repertoire. This is particularly important because RIG-I is the primary sensor of Influenza infections in humans and it is believed that the lack of RIG-I in chickens contributes to its higher susceptibility to highly pathogenic avian influenza (HPAI). We believe that understanding the mechanistic changes which allow the remaining RLRs to sense a broader range of dsRNAs will aid in the development of therapeutics and vaccination strategies to combat avian viral diseases.
AREA 3: Scavenger RLRs
Scavengers are constantly exposed to pathogens during their consumption of carrion, yet they do not suffer from inappropriate activation of the inflammatory response. It is unclear how the immune systems of scavengers are adapted to avoid what would be, for other organisms, a potentially fatal inflammatory response to certain pathogens. Analysis of recently sequenced genomes of several vulture species has demonstrated that there is significant positive selection for genes involved in the immune system, including pattern recognition receptor pathways. However, to date, there has been no characterization of RLRs from vultures (or other scavenger species). By understanding how the conformational landscape and dynamics of RLRs have been adapted in scavengers, we will gain a deeper perspective on the ability to regulate RLR signaling.
Techniques in the Ramsey Lab
- Optical spectroscopy
- Circular dichroism
- Isothermal titration calorimetry
- Differential scanning calorimetry
- Analytical ultracentrifugation
- Microscale thermophoresis
- Differential scanning fluorimetry
- Hydrogen-deuterium exchange mass spectrometry (HDX-MS)
- Liquid chromatography
- Mammalian cell culture
- Confocal fluorescence microscopy
- In-cell transcriptional activation assays